Here’s how L.A.’s Curative Plans to Move Saliva-Based COVID-19 Testing to Other U.S. Cities
Fred Turner, the 25-year-old founder of Curative Inc., is the man behind L.A.'s push to bring universal testing to the region. But, he has bigger plans.
Turner, an Oxford dropout, just landed a deal with the Air Force to test military worldwide and he's now eyeing national expansion for his startup. By the end of this month, the company he started months ago is expected to pump out more than a million test kits a week.
"We are a strange company because our goal is to essentially put ourselves out of business," Turner said.
Turner, who was named UK Young Engineer of the Year at age 17 when he built a DNA machine from his bedroom to figure out why his brother had red hair and he had brown hair, turned his focus to coronavirus in January. He upended his life as head of Shield Bio, which sought to eliminate sepsis, and moved to Southern California after a local venture capitalist connected him with a lab that he could build out for COVID-19 testing.
Curative Inc. was born.

Curative, Inc. Founder Fred Turner is the man behind L.A.'s push to bring universal testing to the region.
For weeks he lived in hotels as he created what has become the backbone of testing in America's second largest city. The company, one of the few saliva-based tests that have gotten emergency approvals from the FDA, has a 10% false negative rate, according to their own non-peer reviewed studies. Turner argues the rate is better than most of the nasal swab testing out there and oral tests, which can be self-administered, are the only way the country can reopen quickly.
He is now operating two labs, one in San Dimas another in Washington D.C., with plans for several more across the nation. Meanwhile, the rest of the country is watching to see if Los Angeles' promise of universal testing meets the expectations with some already complaining about the difficulty of securing an appointment.
dot.LA: Curative is responsible for 95% of Los Angeles COVID-19 tests. Can you meet the demand for universal testing?
Curative, Inc. Founder Fred Turner: We will be able to fulfill demand. There's more infrastructure that needs to be put in place. With the collection site, the mayor's office has been working hard to scale those up and make sure that the collection site infrastructure is in place. On the lab side, we definitely have enough capacity to serve the area.
In (the San Dimas) lab we have capacity for about 20,000 per day. We're going to be scaling this lab up to 30,000 over the next week or so, eventually, probably slightly more than that.
Ultimately, the goal is to do as many tests as we need to reopen the country and I think we've had many different estimates of how many that might be. I've heard estimates (that it will take) as high as five million tests a day to reopen, but the current supply chain is nowhere near going to be able to keep up with that. The next goal we are shooting for is a million tests a week by about mid-May, end of May.
If we're testing more people will that strain the turnaround time? Right now, I understand it's about 24 to 72 hours. Can you take it down to several hours or even minutes?
We are always aiming to push it down. I think it can be pushed under 24 hours, but we tend to focus more on scaling it up at that point. I think 24 hours is around optimal as you bring on more capacity. We're obviously being careful to match the capacity coming into the lab against the turnaround.
Curative is one of the few companies that the FDA has provided emergency approval for use of a saliva test. Do you think this is the answer to universal testing nationally?
Oral fluids is the technical term, which is slightly different from saliva. Our protocol involves having the person cough first, which releases virus from the upper and lower respiratory tract. Some of that is then caught in the saliva, and also viruses in the saliva. The idea is you're effectively sampling multiple sites at once, which we think gives a slight increase in the sensitivity of the testing.
Self-collected sampling has got to be the way forward. There's just no way that we can do a million nasal pharyngeal swabs, or we call them 'brain swabs,' it's just not practical. We don't have the medical staff, and as a country we just can't do that many swabs. And so the way forward is going to be self collection. That's the only thing we can do at scale.
Covid-19 Oral Fluid Test Kit Instructionswww.youtube.com
Curative recently secured a contract with the Department of Defense. What's next?
We haven't settled on the exact number, but we will be in a large number of states. That is the plan, building out the infrastructure for this kind of testing. We can take the L.A. model and scale it across the country. We have a version of the drive-thru software that other cities and states can take. We have all the training material. We have a team that fly around the country setting up these drive-thru sites. We have really tried to make a plug-and-play package where cities and states that want to launch drive-thru testing, we have everything they need.
Is Los Angeles a testing ground and a proving ground for national efforts?
Yeah, I think Mayor Garcetti has definitely demonstrated what can be done if the city moves very quickly to build out the infrastructure, I do think the drive-thru testing, walk through testing is going to be an essential component of this.
It's just much faster to get the samples to the lab than shipping it out and then shipping it back again. The infrastructure piece is critical for getting these tests out there and we're working with several other states now on building out a similar infrastructure. Some of them have existing programs that we would be plugging into and expanding, and some of them are looking to roll out their own similar programs. You can test a lot more people with the oral tests with far fewer staff.
What are the challenges with the supply chain in terms of making your tests more widely available?
We need more plastic, more robots, more people. Most of the components that go into these tests are just already maxed out in their production capacity. And so we've been throwing online a bunch of internal production, such as injection molding to make our swab kit tubes, as well as bringing on other sources of similar materials that are not being used for COVID-19 testing and validating them for COVID. So for the swabs, for example, we use a swab type that is usually used for testing clean rooms. It works just as well for COVID testing, but nobody else is using it for that purpose.
We don't want to be competing with other people, stopping them from getting access to resources. We want to bring on new supply so it's not a zero- sum game, and the total amount of tests will increase. But, I think distribution is going to be a bigger bottleneck over the next month. Los Angeles has done a fantastic job in building out infrastructure and collection. That needs to happen now across the whole country.
What would it take to meet national demand?
It would take a network of multiple labs that we are calling gigalabs that can process 50,000 to 100,000 tests a day across the country and a significant scale up in the production of the physical components that go into the tests. We've been investigating lab sites in a few states, something central like Texas or Colorado.
Are you concerned that you have too high of a false negative rate at 10%?
All COVID tests will have false negative rates. In our studies, we demonstrated that the sensitivity is at least as good if not better than the nasal pharyngeal swab tests. So, there will definitely be false negatives as there will be in every test, but we think the data supports us having a low rate of false negatives. The sensitivity is about 90%.
When you include all patients, the nasal pharyngeal came in at about 79%. A new (non-peer reviewed) Yale study shows a higher sensitivity from saliva and all fluids than the nasal pharyngeal. I don't think we have the evidence to say that we're better yet, but we have the evidence to say that we're at least equivalent. The ease of doing oral tests and the accessibility is obviously significantly better. We're not using PPE. We're not exposing healthcare workers. It's just a much easier test to roll out that people can test themselves.

At one point, Curative was attempting to roll out at-home testing and then had to pull back after FDA warnings. Will you be making home testing kits again?
I can't give a time estimate but we are actively working with the FDA right now. I think their concern is "can people adequately collect the sample, or will they do it wrong, and then get a negative test result," which is incorrect. Our plan is to use telemedicine observation. What we've seen in our clinical studies is that if you give people the chance to do it wrong, they will do it wrong. In fact when you have somebody observing, they read the instructions better and they collect a better sample. We're still working on the pricing and potentially looking at whether there is federal or state reimbursement for some of that testing and the potential of billing insurance.
Tracing technology has been promising. Do you have any plans to tie tests to these applications to testing?
We've had several conversations with contact tracing apps and we continue to work in collaboration with public health departments in their contract tracing efforts. Having the test is obviously one thing, but you have to use the information to actually stem the tide of COVID infection. And so, it is an essential step in working closely with various people.
I do like the app-based models where you obviously want to maintain people's privacy, but having people opt-in to share their location history if they test positive so you can contact nearby people.
What does Curative do once there's a vaccine? Are you obsolete?
The U.S. needs to maintain some kind of spare capacity so that next time we are not having to build all of this during a pandemic. But we are a strange company because our goal is to essentially put ourselves out of business. Ultimately, and I say this to everyone we hire, we don't know how long this will go on for. We want to provide as many tests as needed, but the goal is to end COVID and not be doing COVID testing anymore.
Curative took off in March when you arrived in Los Angeles from Silicon Valley, how has the journey been for you personally?
I don't have very much free time, but it's been a lot of fun to be able to work at this pace and have the support of people like the city, the mayor's office to really just do what is needed to scale this up. When we work with suppliers, and we say we're doing COVID testing, they just move heaven and earth to make things happen. It's been really inspiring to be a part of that push forward.
We are just pushing as hard as we can to scale up as fast as we possibly can and bring on as much testing as we can. The city opening up testing to everybody is kind of a culmination of that. We're making about 50,000 kits a day right now and we want to make that as widely available as we can. I would like to get a little more sleep.
The interview has been edited for clarity and brevity.
Correction: An earlier version of this story stated that Curative's saliva tests have a 10% false positive rate. In actuality, the tests have a 10% false negative rate.
Do you have a story that needs to be told? My DMs are open on Twitter @racheluranga. You can also email me.
- coronavirus - dot.LA ›
- coronavirus-tests - dot.LA ›
- curative-inc - dot.LA ›
- Here's How Curative, Inc, Plans to Roll Out Coronavirus Testing to ... ›
- FDA Approves Curative Inc's COVID-19 Test - dot.LA ›
- How One Startup is Preparing for Coronavirus - dot.LA ›
- COVID-19 Test Kit-Maker Curative Inc.is Struggling to Ramp Up - dot ... ›
- How Curative Inc. Plans to Test the U.S. for Coronavirus - dot.LA ›
- Founders and Investors Do Not Share Wall Street's Optimism - dot.LA ›
- Founders and Investors Do Not Share Wall Street's Optimism - dot.LA ›
- Snap Brings Meditation to the Masses - dot.LA ›
- Snap Brings Meditation to the Masses - dot.LA ›
- Curative Founder Fred Turner on Next Steps For COVID Testing - dot.LA ›
- Curative Inc. Brings COVID-19 Testing Kiosks to Los Angeles - dot.LA ›
- Los Angeles Opens COVID Testing Kiosk at Union Station - dot.LA ›
- Los Angeles Opens COVID Testing Kiosk at Union Station - dot.LA ›
- COVID Testing Kiosks Are Heading to LAX - dot.LA ›
- Healthvana Sends Vaccination Records to Apple, Google Wallet - dot.LA ›
- Curative COVID Tests Carry Risk of False Results, FDA Warns - dot.LA ›
- Curative's COVID Tests Will No Longer Be Used in LA County - dot.LA ›
- Curative will Administer Vaccines at Dodger Stadium - dot.LA ›
- A New Coronavirus Vaccine Wants to Take on More Variants - dot.LA ›
- XPrize Awards Winners of COVID-19 Test Competition - dot.LA ›
- The Labs Tasked with Making LA's COVID Testing Mandate Work - dot.LA ›
- Dodger Stadium Reopens Curative COVID Test Site - dot.LA ›


 Tarryn Marcus, left, and Zach Boldyga are leading a volunteer-driven effort to build a COVID-19 testing location database. Photos courtesy of Tarryn Marcus
Tarryn Marcus, left, and Zach Boldyga are leading a volunteer-driven effort to build a COVID-19 testing location database. Photos courtesy of Tarryn Marcus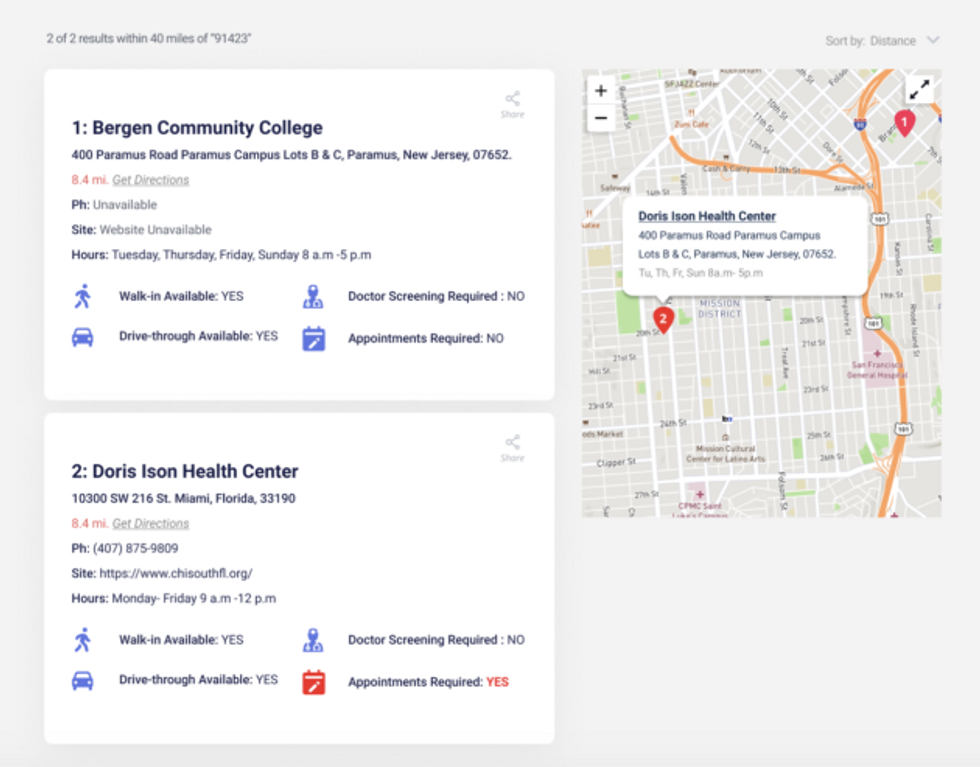 A screen grab showing the information available for a testing location.Get Tested COVID-19 Image
A screen grab showing the information available for a testing location.Get Tested COVID-19 Image