As the states of California, Oregon and Washington begin strategizing on their regional plan to re-open their economies and control the future spread of COVID-19, a crucial question remains: How can businesses ensure their employees remain virus-free now and in the future?
For the thousands of western U.S. businesses seeking to safely bring their employees back to work, and tens of thousands of residents who desperately want and need to return to their jobs, a robust supply of COVID-19 tests, coupled with a systematic COVID-19 testing strategy supported by evidence-based guidelines will be key to success.
To date, the country has faced testing shortages, confusion about the various testing types, and lack of clarity around who should or can have access to tests. However, that does not mean we can't course-correct by thoughtfully laying the necessary roadmap for more transparent and effective testing guidelines as we look to reopen our economy.
The first step is a clear understanding of the current testing options available. The two predominant tests on the market are viral tests and serum antibody tests:
- Viral, or PCR, tests determine if an individual currently has COVID-19 by testing for the virus itself through a swab of the nose or throat. These tests indicate whether the person is contagious to others, even if they are asymptomatic. Viral tests are complex and can require at least 24 hours to perform and report. The PCR test is considered the gold standard given its 90%+ accuracy rate and ability to detect even low levels of virus.
- Antibody tests determine whether an individual has been infected with the virus and has developed an immune response (antibodies) against the virus. Antibody tests use a blood sample and can be processed relatively quickly, yet they will not identify people with an early infection, nor can they tell whether an individual is no longer shedding the virus and therefore *no longer contagious.
Both types of tests can be delivered as laboratory tests or point-of-care (POC) tests, the latter having the goal of quick turn-around and broad community delivery. However, none of the companies marketing POC tests have published accuracy data based on patient testing yet.
So where does that leave businesses looking to get their workforce back up and running? Businesses must establish protocols for appropriate testing of employees to confirm that they are virus-free before returning to work. Taking someone's temperature is NOT a valid proxy for determining whether an individual is "healthy" nor is an antibody test alone. Instead, we need to develop a clear decision tree that includes the two different types of testing.
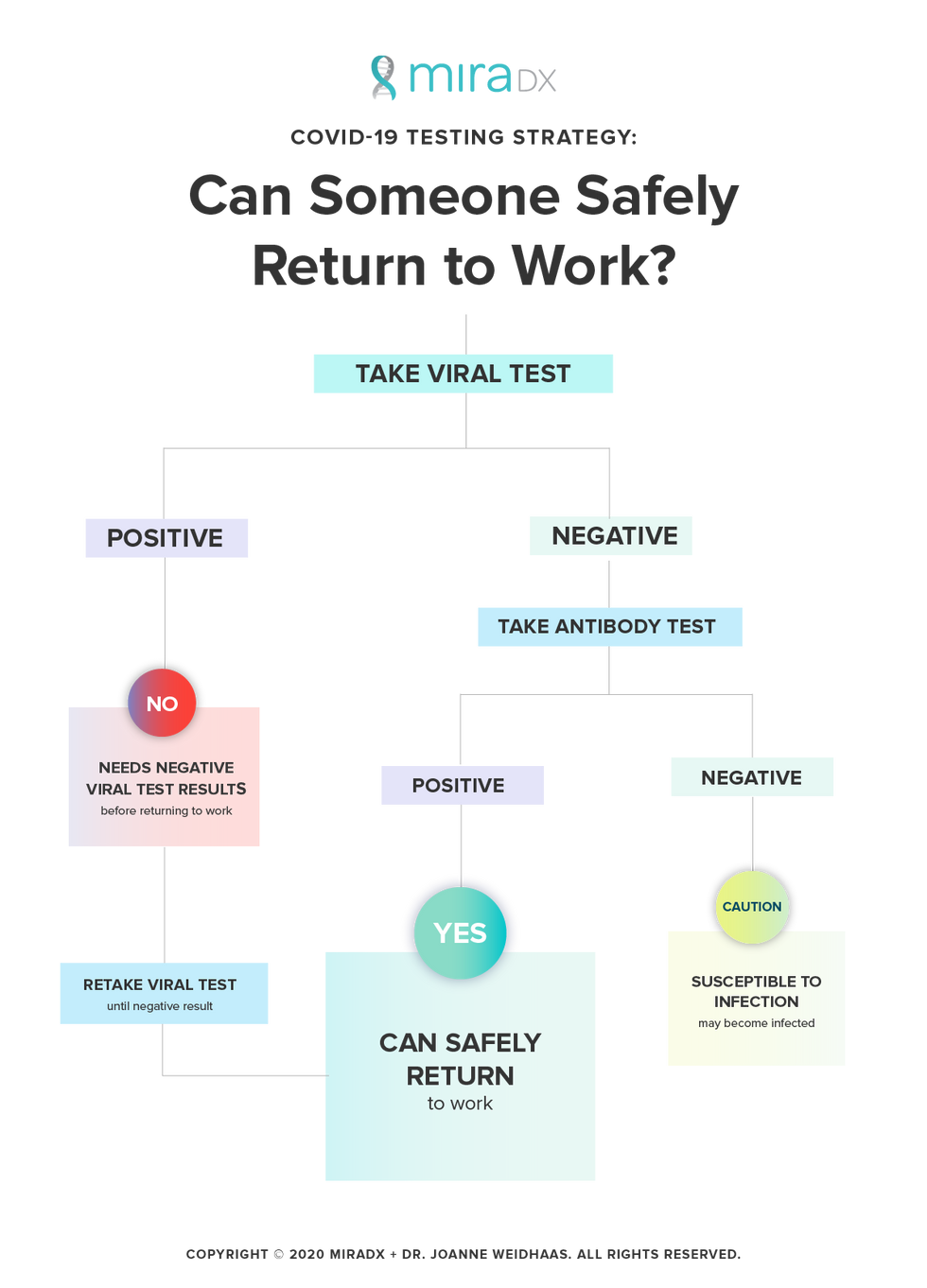
Photo courtesy of Joanne Weidhaas
Companies should institute a multi-step COVID-19 testing protocol:
1) Any person who previously tested positive must receive a negative viral clearance test before returning to work.
2) Any person who has no confirmation of prior infection should receive a viral test first. If they are positive for the virus, they must be retested until they receive a negative result. If they are negative for the virus, they should then have an antibody test to determine whether they were previously infected.
If negative for virus and positive on the antibody test, they can also safely return to work. If negative for the virus and negative on the antibody test, they should know they are susceptible to infection if they return to work.
For those who are still susceptible, one option is to allow them back to work, but develop a prospective viral screening protocol. These screening tests could be on a volunteer and rotating basis through the workforce, with perhaps 10%-20% being tested each week. Anyone with symptoms also should be sent home and receive immediate viral testing.
The federal government should be taking action to coordinate testing supply, help direct it to the highest priority populations and subsidize the cost of testing. Business leaders should coordinate efforts to establish testing protocols and sharing of testing resources for the workforce that reflect the most current science to ensure that workers can safely return to, and stay at, work.
Viral testing is the only established method to confirm if an individual is virus-free and therefore safe to return to work. At MiraDx, we are conducting PCR testing for COVID-19 and are seeing many individuals continue to be contagious for weeks after their last symptoms. This is significantly longer than the current CDC guidelines that indicate that individuals are "clear" to return to work 72 hours after fevers and symptoms are gone. We are completing a larger study on this issue and will report results soon.
Dr. Joanne Weidhaas, MD, PhD, MSM is the co-founder of Los Angeles-based molecular genetics company MiraDx, the founder of MiraKind.org, and currently a professor and vice-chair in the department of radiation oncology at UCLA.
*This story has been corrected to say that if you are no longer shedding virus, you are no longer contagious. It previously and improperly said the opposite.
- The COVID-19 Crisis is Creating a New Biotech Culture in L.A. - dot.LA ›
- Guest Column: Here's How Businesses Can Make Sure their ... ›
- Here's How Businesses Can Make Sure their Employees Are Virus ... ›
- How Will Offices Change After Coronavirus? - dot.LA ›
- How Will Offices Change After Coronavirus? - dot.LA ›
- Techstars' Advice to LA Entrepreneurs - dot.LA ›
- Techstars' Advice to LA Entrepreneurs - dot.LA ›
- If a Competitor Calls, What Should You Do? - dot.LA ›
- As the Pandemic Recedes, Get Ready for Office Awkwardness - dot.LA ›

