Health Tech Has a Higher Bar To Meet Before It Hits The Market—And It Starts With the FDA
This is the web version of dot.LA’s daily newsletter. Sign up to get the latest news on Southern California’s tech, startup and venture capital scene.
Earlier this week, West Hollywood-based startup Pearl announced that its Second Opinion product had become the first AI-enabled device cleared by the Food and Drug Administration to read dental x-rays. Using the power of artificial intelligence, Second Opinion is meant to help dentists find maladies they’d otherwise miss through the eye test.
Getting FDA clearance is not easy, especially because Pearl had to prove its device could detect a variety of dental conditions (most medical devices have to prove only one capability). Pearl’s CEO, Ophir Tanz, said it was a multi-year undertaking that entailed a 4,000-page clinical trial report.
There’s a stark juxtaposition between a fast-paced tech world, where investors strive for needle-moving investments and a painstakingly slow regulatory process required to greenlight such technologies. Perhaps things would be easier if the FDA only regulated food and drugs; instead, it’s in charge of overseeing an ever-growing universe of devices and apps that blur the line between consumer technology and medical equipment.
This is especially true as the pandemic has ushered in a new wave of health startups trying to address inefficiencies in the current system—from mental health apps built as a supplement or alternative to antidepressants and therapy, like Santa Monica-based Headspace Health, to virtual reality tools meant to provide at-home treatment for chronic conditions, like Van Nuys-based AppliedVR. (Both of those startups have received FDA approval for their products.)
By its own admission, the FDA isn’t fully equipped to regulate some newfangled technologies, as that would require a clear understanding of how the technology works and a set of guidelines governing them. In 2018, then-FDA Commissioner Scott Gottlieb announced that that agency would begin creating better frameworks to address some of these shortcomings—but by all indications, progress is slow.
As a result, even if those technologies make it to market, it’s difficult to ask clinicians to adopt them. What happens if they rely too heavily on a relatively unproven platform, and its faults get them sued for malpractice? What if a doctor disagrees with a piece of technology?
That’s not to say the FDA should adopt the for-profit tech world’s fast-and-loose style. After all, there’s a reason regulatory processes are exceptionally deliberate and thorough. In a world where social media platforms can be used to meddle with democracies, imagine what a piece of health technology can do to the human body if not fully vetted and allowed to go awry. — Keerthi Vedantam
Gaming Unicorn Scopely Invests $20 Million In a New Studio
The investment is part of Culver City-based Scopely’s plan to expand its “ecosystem” of studios that it has built, bought or backed around the world. The goal is to grow Scopely’s portfolio by pairing its expertise with specialized teams from internal and external studios.
Venture Firm Chapter One Has a New Crypto-Focused Incubator
After raising a $40 million fund in December, the Los Angeles-based venture capital firm has launched a crypto-focused incubator called Chapter One Studios. The six-month program starts in April and will provide three startups with a $1 million investment and the option to work out of Chapter One’s L.A. office.
TikTok Launches Feature To Help Musicians Monetize Songs
Social media giant TikTok has turned little-known musicians into mainstream stars. Now, the Culver City-based company is giving artists a new way to monetize their music through the video-sharing app.
Slingshot Aerospace Raises $25 Million
The El Segundo-based space data and analytics startup has expanded its Series A round to help grow its team and continue work on its flagship product, a collision avoidance system for space vehicles called Slingshot Beacon.
Rivian Stock Tanks on Missed Earnings Expectations
The Amazon-backed, Irvine-based electric automaker saw its stock slump as much as 13% in after-hours trading Thursday after the company announced it had missed earnings expectations—by a lot.
Office Hours: Factors To Consider When Broadening Your Startup
Many startups that have established a strong foothold begin with narrow markets and then have to decide if and when the time is right to broaden their product and expand their market. So, how do you know when, and how, to make that move? Zillow co-founder and dot.LA Chairman Spencer Rascoff offers some advice.
🎧 Listen Up: 'Gotham Gal' Joanne Wilson on Telling Good VCs From Bad
On this episode of the LA Venture podcast, angel investor Joanne Wilson discusses her journey from department store management into cannabis investing.
What We're Reading Elsewhere...
- Pacific Palisades-based low-carbon fuel maker WasteFuel partners with Maersk.
- Hawthorne-based Astrolab unveils its lunar rover design.
- Disney becomes the latest U.S. company to pull out of Russia.
- L.A.-based entertainment rights and finance platform Rightsline acquires IP management software company REAL Software Systems.
How Are We Doing?
Notice some changes to the daily newsletter? We heard you and we're working to make the newsletter more informative, with deeper analysis and more news about L.A.'s tech and startup scene. Let us know what you think in our survey, or email us!
- Investors and Health Tech on the Front Lines of the Pandemic - dot.LA ›
- New Technology for Seniors Is Allowing Them to Age at Home - dot.LA ›
- AppliedVR's Virtual Pain Therapy Platform Gets FDA Approval - dot.LA ›
- Dental Startup Pearl Gets FDA Clearance for AI-Powered X-Ray ... ›
- Startups Can’t Cure America’s Preventative Health Care Failings - dot.LA ›
- Top L.A. Proptech Companies in 2021 - dot.LA ›


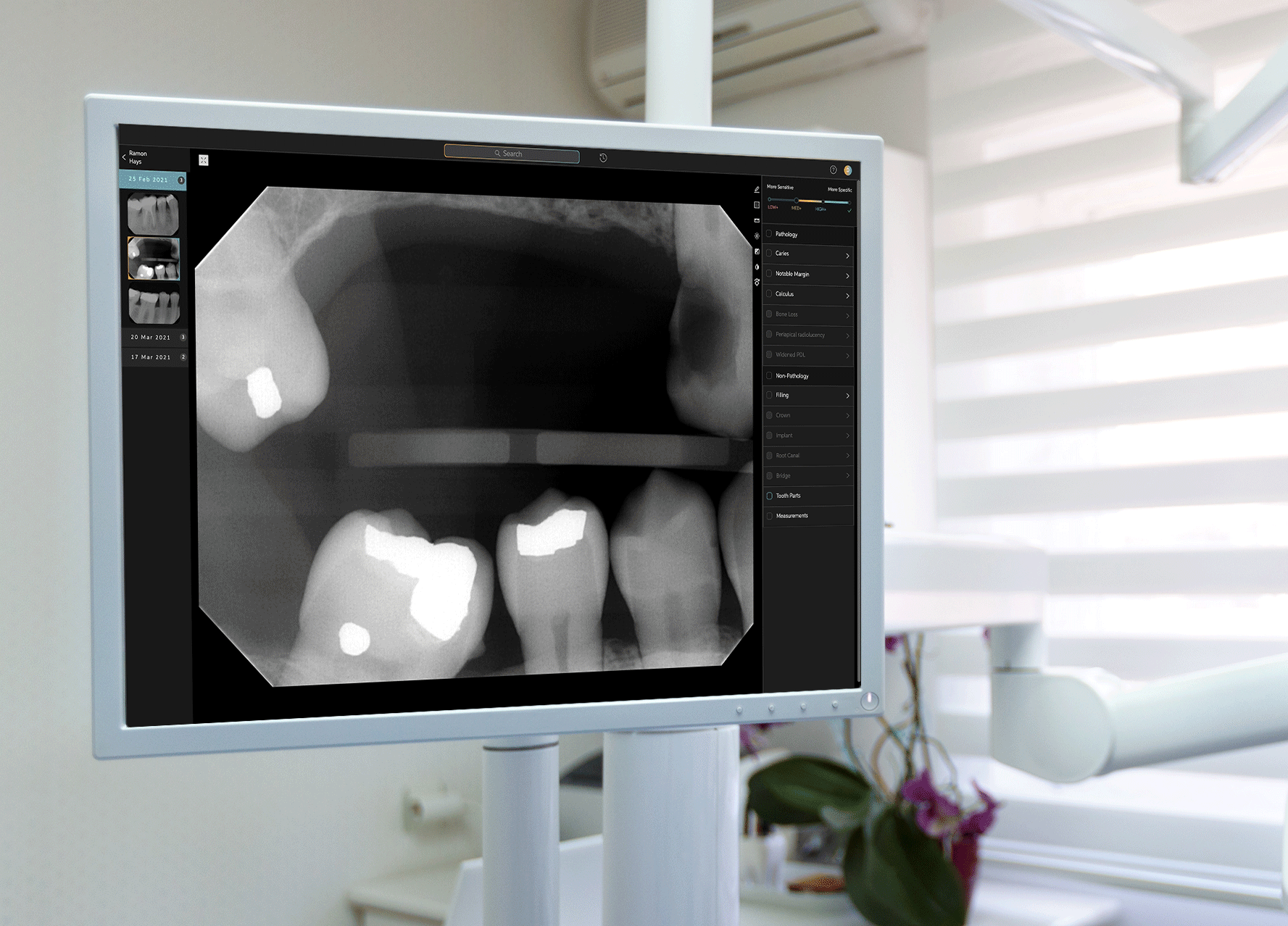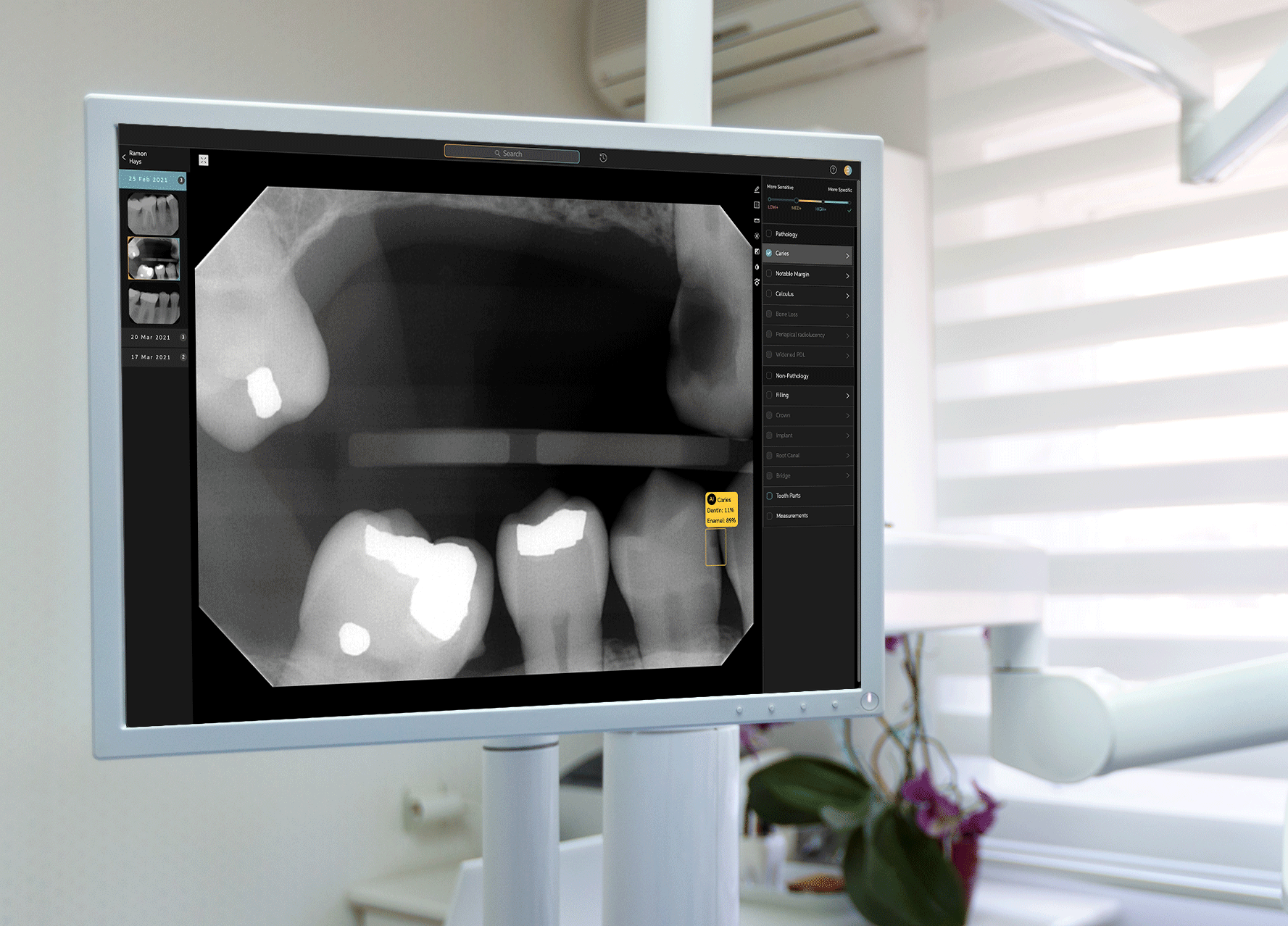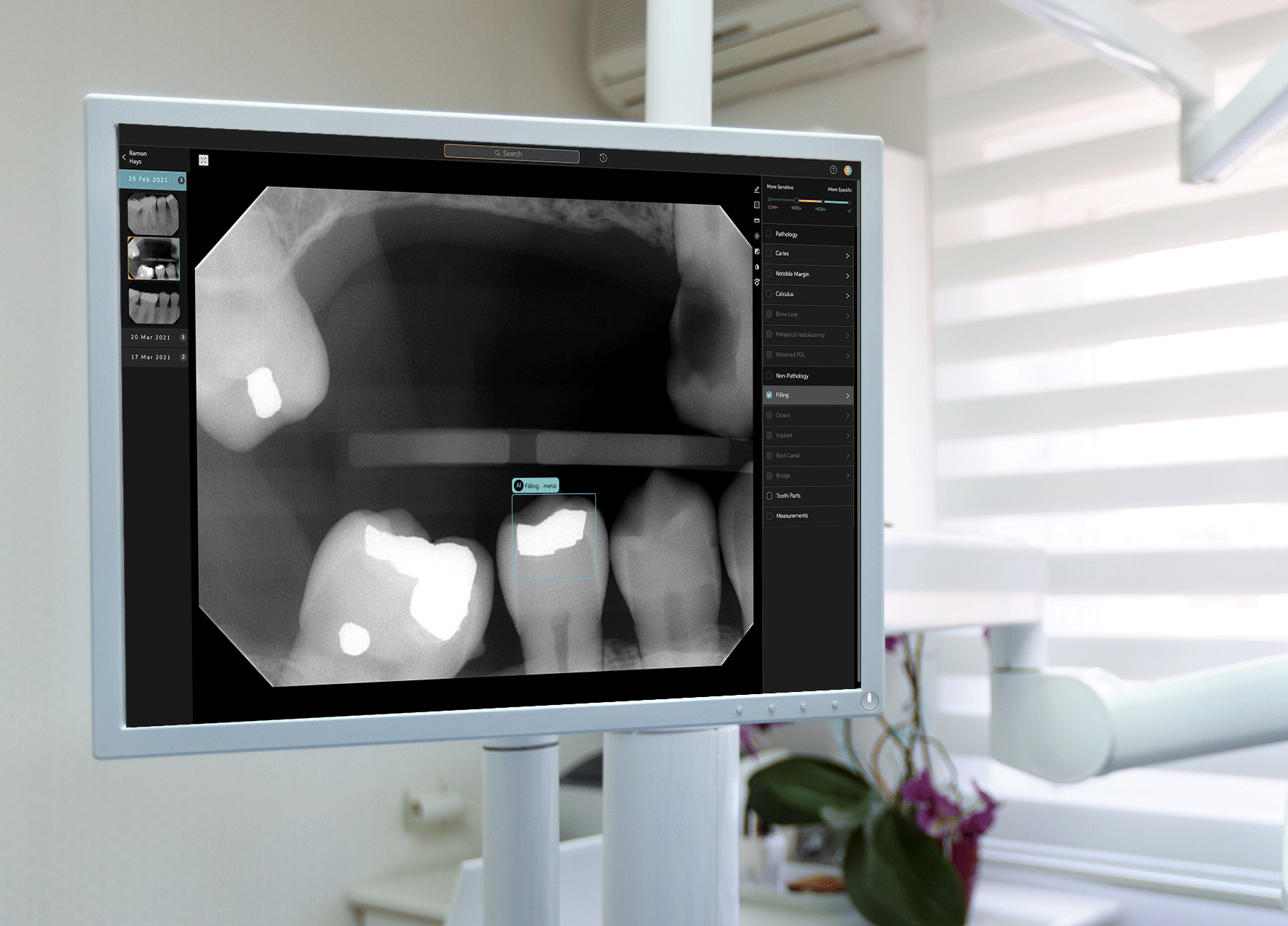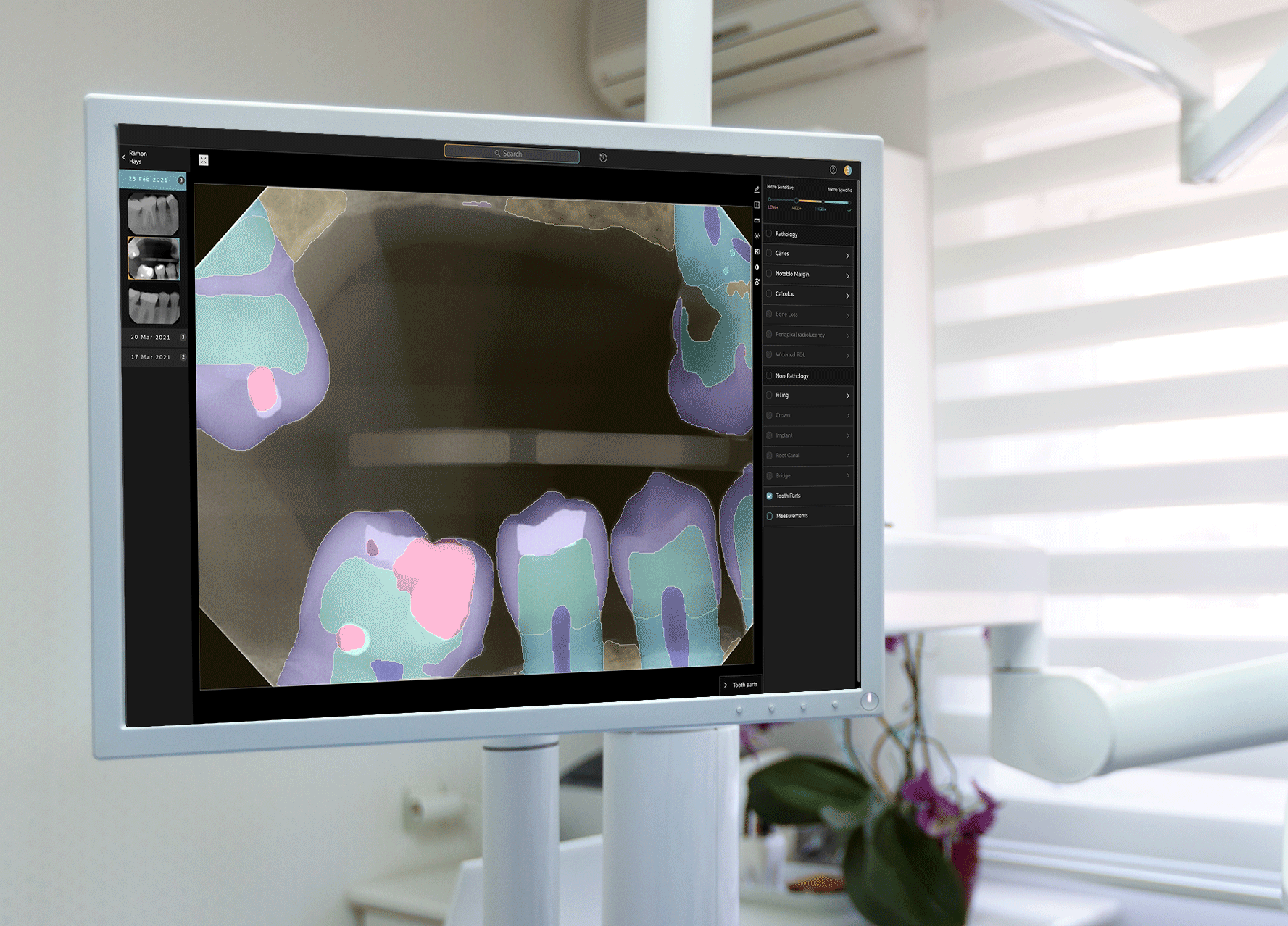 Pearl’s AI program examine teeth.Courtesy of Pearl
Pearl’s AI program examine teeth.Courtesy of Pearl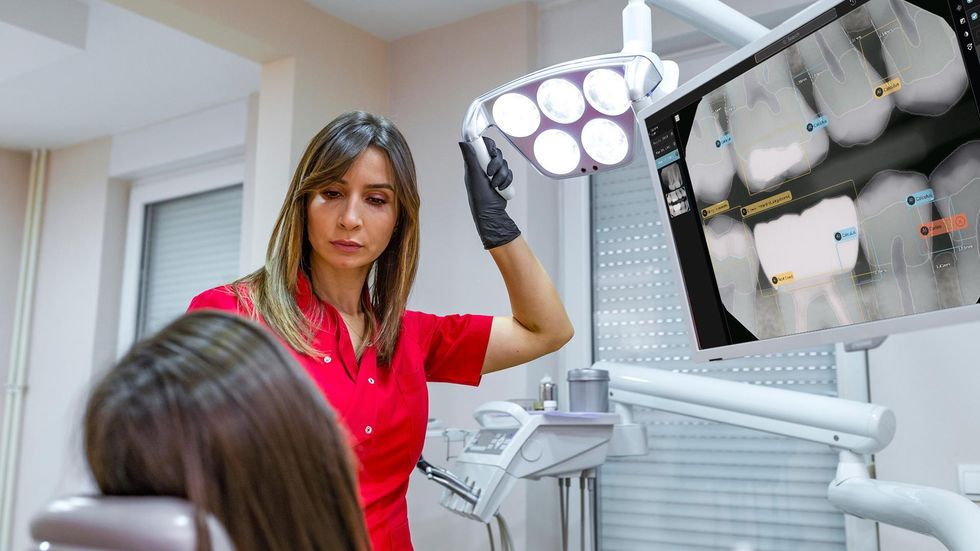 A patient goes under the lights in the dentist’s office.Courtesy of Pearl
A patient goes under the lights in the dentist’s office.Courtesy of Pearl