Vaccine Pause Sends LA Officials Scrambling
Keerthi Vedantam is a bioscience reporter at dot.LA. She cut her teeth covering everything from cloud computing to 5G in San Francisco and Seattle. Before she covered tech, Keerthi reported on tribal lands and congressional policy in Washington, D.C. Connect with her on Twitter, Clubhouse (@keerthivedantam) or Signal at 408-470-0776.
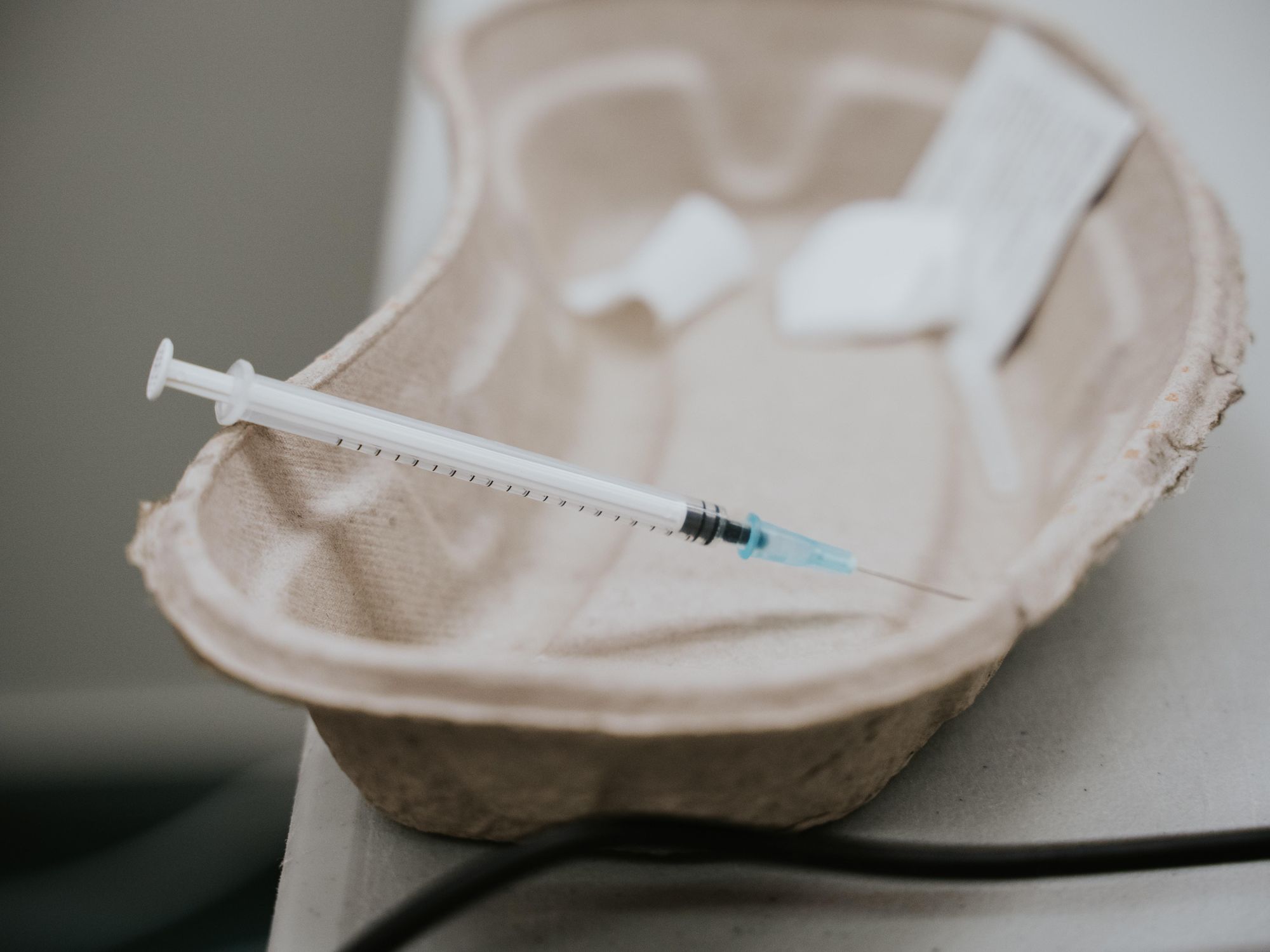
Los Angeles is scrambling to deal with the federal health officials' call for a pause in the use of single-dose Johnson & Johnson vaccines that have helped speed up local inoculations.
L.A. Mayor Eric Garcetti said city-run vaccination sites had more than 3,000 patient appointments scheduled for Tuesday that will now receive a first dose of Pfizer, instead of the planned Johnson & Johnson vaccine. It comes two days after Los Angeles County opened vaccinations to everyone 16 and older.
In Long Beach, officials said those with an appointment at mobile clinics where the Johnson & Johnson vaccine was scheduled to be given will be contacted and offered either a Pfizer and Moderna vaccination. So far, no local cases of the rare blood clotting that forced federal officials to stop use of the vaccination have been reported, Long Beach officials said in a statement.
The move comes after the Center for Disease Control (CDC) and the Food and Drug Administration (FDA) reported six cases of blood clots among individuals who received one of the 6.8 million Johnson and Johnson vaccines distributed nationally.
Already, 900,000 of the vaccines have been distributed to people in California, making up roughly 40% of all vaccinated adults in the state. But, Gov. Gavin Newsom said on Twitter Tuesday that only 4% of the state's current supply is Johnson and Johnson.
In a joint statement, the CDC and the FDA said the recommendation to pause the vaccines was made "out of an abundance of caution." The agencies will use this time to understand and prepare treatments for these blood clots.
"The system is working as it should," said Shira Shafir, a public health professor at UCLA. "If we suspect something might be happening, then we do evaluate whether or not we have enough information."
Shafir said the CDC and FDA may decide to update recommendations to better avoid the serious blood clots. Neither agency has determined that the vaccine does cause the blood clots.
The move will largely impact vulnerable populations, such as those who are homebound or live in rural areas, or who cannot take time off work.
That's because the Johnson & Johnson vaccine did not need to be stored in sub-zero temperatures like Moderna and Pfizer vaccines and is administered in a single shot.
Get Out the Shot, a 400-person volunteer program in Los Angeles dedicated to booking vaccine appointments for people who may not have proper documentation or have a language barrier, are informing people if there is a vaccine change.
"One of the big concerns with a lot of the people that we're helping is the wage loss that accompanies time off in order to get to that vaccine appointment," said founder Liz Schwandt. "So we don't want to have to double someone's time that they have to spend away from providing care for their families or from working."
But, Schwandt said, smaller community clinics who cannot pivot quickly to a different vaccine are cancelling appointments, making it difficult for people who took time off work to recoup those lost wages.
It is unclear if this will affect California's goal of opening its economy June 15, though the California Department of Public Health said in a press release, "we do not expect a significant impact to our vaccination allocations."
Every state is allotted a certain number of the Johnson & Johnson, Moderna and Pfizer vaccines to distribute. About 4% of the state's vaccine supply for the week came from Johnson & Johnson. It's unclear what effect that will have for people getting vaccines in the future.
Garcetti said Los Angeles has received about 60,000 doses of the Moderna vaccine and 56,000 of the Pfizer vaccine this week.
A facility in Baltimore that was making AstraZeneca vaccines (which has also been linked to rare blood clots and is not authorized for use in the U.S.) is being investigated after it mixed up ingredients meant for the Johnson & Johnson vaccines. The New York Times reports the vaccines in circulation are not from that facility.
Still, Shafir said the news shouldn't deter those hesitant to receive the vaccine from scheduling an appointment. The AstraZeneca vaccine (which has also been linked to rare blood clots and is not authorized for use in the U.S.) and the Johnson & Johnson vaccine are adenovirus vector vaccines, while Moderna's and Pfizer's are mRNA vaccines.
Though the two effectively operate the same way once in the body, adenovirus vector vaccines introduce a weak version of the virus while mRNA vaccines introduce a peculiarly shaped protein — called the spike protein — into the body. It's similar to the coronavirus spike protein. There is no evidence that mRNA vaccines, like the Moderna and Pfizer vaccines, have produced blood clots.
"The chances someone is going to get infected with COVID and get seriously ill as a result of being infected with COVID is still multiple times higher than the chance that they will develop this very serious complication as a result of COVID vaccination," Shafir said.
This story has been updated.
- Where to Find a COVID Vaccination Appointment - dot.LA ›
- Pfizer, Johnson & Johnson or Moderna? How to Find the Vaccine You Want - dot.LA ›
Keerthi Vedantam is a bioscience reporter at dot.LA. She cut her teeth covering everything from cloud computing to 5G in San Francisco and Seattle. Before she covered tech, Keerthi reported on tribal lands and congressional policy in Washington, D.C. Connect with her on Twitter, Clubhouse (@keerthivedantam) or Signal at 408-470-0776.



 Image Source: Revel
Image Source: Revel