This is the web version of dot.LA’s daily newsletter. Sign up to get the latest news on Southern California’s tech, startup and venture capital scene.
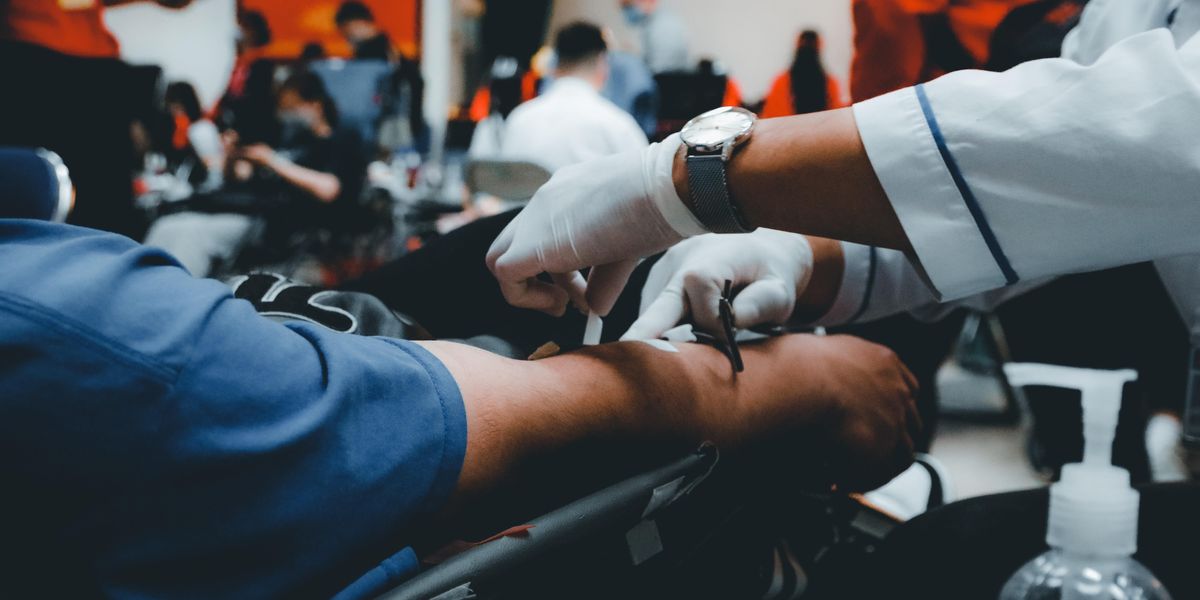
When I first started looking into the matter of diversity in clinical trials, I almost didn’t understand the issue. After all, everyone’s blood cells and vital organs look virtually the same, right? So why would the drugs that treat our ailments need to be tested across different demographic groups?
Perhaps the best example of the faults with this kind of thinking is the sleep medication zolpidem, more popularly known by its brand name Ambien. Women were severely underrepresented in zolpidem’s clinical trials prior to its release in 1992; as a result, they were more susceptible to impaired alertness in the morning that may have caused driving accidents. It wasn’t until 2013 that the FDA revised its recommended dose of the medication for women.
The National Institutes of Health mandated that women must be included in clinical trials in 1993. But the problem persists. Today, those already sitting at the margins of our health care system—the poor, disabled and people of color—are still chronically underrepresented in trials, despite often needing care the most.
Clinical trials are the painstaking and extremely critical processes that the FDA requires to determine if a drug is safe and efficacious. They are extremely thorough—90% of drugs fail in trials after undergoing years of research and development—and often require participants to be involved during work hours and drive to clinical trial sites. This automatically makes it harder for people who work multiple jobs, who don’t have childcare available to them or who can’t afford to travel long distances to participate. As a result, we often miss crucial lifestyle data that can determine how drugs are affecting peoples’ day-to-day lives (like how drowsy they are in the morning). That’s compounded by the fact that the clinical trial dropout rate hovers around 30%.
Bringing greater access and transparency to clinical trials is one the goals of Newport Beach-based VivoSense, which announced a $25 million Series A funding round on Wednesday. The startup’s data collection technology collects and organizes data from wearable biometric sensors, which allow drugmakers to see how their therapeutics affect patients in real-world settings rather than just clinical trial sites.
The pandemic alleviated some of these bottlenecks by forcing pharmaceutical companies to adapt to social distancing measures. El Segundo-based MedVector created a shoebox-sized device that can collect biologic information at a nearby doctor’s office, instead of asking participants to visit trial sites. Another El Segundo-based startup, Lightship, constructs virtual clinical trials for pharmaceutical companies. Others, like Los Angeles-based Topography Health, recruit participants for trials that could create a more diverse sample size.
It’s early days as far as telling whether these startups, and the measures they’re pursuing, will demonstrably improve the diversity of the clinical trials that vet the drugs we rely on. But given medicine’s long and checkered history of overlooking the most vulnerable groups, it can’t be worse than before. — Keerthi Vedantam
E3 Gaming Conference Won’t Happen At All This Year
The rumors turned out to be true: The annual blockbuster video game conference typically held in Downtown Los Angeles, is once again entirely canceled this year.
Slingshot Aerospace Lands $25 Million Space Force Contract
Fresh off securing a $25 million funding round earlier this month, Slingshot Aerospace has inked a new $25.2 million contract with the U.S. Space Force that will see America’s newest military branch use two of the El Segundo-based startup’s flagship products.
Collectors Raises $100 Million and Rebrands
The Santa Ana-based company—which is backed by athletes such as NBA star Kevin Durant, NFL great Larry Fitzgerald and former U.S. Open tennis champion Andy Roddick—lets collectors buy, sell and authenticate sports trading cards, rare coins and more online. Its new funding round values the company at $4.3 billion.
Microsoft-Activision Merger Faces Scrutiny From US Senators
Activision Blizzard’s $69 billion merger with Microsoft is being challenged by four U.S. senators, who are urging the Federal Trade Commission to reconsider approving the buyout because it wouldn’t benefit workers.
🎧 Listen up: Impulsum Ventures’ Ed Wilson on Embracing Failure
On this episode of the LA Venture podcast, Impulsum Ventures co-founder Ed Wilson talks about building his firm and the development studio that serves its portfolio companies, along with what skateboarding taught him about how to try implausible things.
What We're Reading Elsewhere...
- Space factory company Hadrian raises $90 million.
- Blue Origin launches its fourth manned space mission from West Texas.
- Electric vehicle maker Fisker racks up 40K reservations for its Ocean SUV.
- Romeo Power employees begin migrating to its new location in Cypress.
- Product placement adtech Ryff partners with Insight TV to insert ads into the free, ad-supported network's offerings.
----
How Are We Doing? We're working to make the newsletter more informative, with deeper analysis and more news about L.A.'s tech and startup scene. Let us know what you think in our survey, or email us!


